Medical interventions are made simpler and more effective by medical equipment. They include anything from catheters and tongue depressors to cardiac pacemakers and surgical lasers. However, developing and producing some medical equipment and devices is one of the most difficult processes, requiring exacting specifications and high-quality standards.
In this article, we highlight the medical device development process, analyzing its phases, and challenges. We’d also discuss considerations and tips for manufacturing medical products.
What Is Medical Device Development?
The process of transforming a medical device into a market-ready product is known as medical device product development. This intricate journey involves multiple phases that streamline the conversion of the device from its initial state to a functional tool within the healthcare sector.
Engineers specializing in medical device development must adhere to a structured framework of stages. Compliance with rigorous regulatory requirements is essential, alongside detailed documentation of their activities to uphold quality benchmarks and facilitate future replication.
Essential Stages of Medical Device Development: A Comprehensive Guide
The fabrication of medical equipment demands meticulous attention to detail and accuracy to meet their intended functions effectively. Consequently, the development timeline of a medical device must adhere to specific crucial stages. Here’s a breakdown of how to craft a medical device through its essential phases.
Phase 1 – Ideation, Conceptualization, and Risk Assessment
The initial stage is expected to span approximately one to two months. The commencement of medical device development involves meticulous planning, extensive research, and accurate documentation. To distinguish this process, it is essential to incorporate opportunity and risk assessments, which play a pivotal role in determining the project’s advancement or potential impediments in subsequent phases.
Navigating the complexities of medical device creation necessitates meticulous consideration of your requirements, which in turn underpin your risk analysis. Establishing a comprehensive development strategy for your medical device is imperative. Typically, the primary phase of a medical device development strategy encompasses the following steps:
- Clearly define the intended purpose of the medical product or device.
- Conduct a market analysis to ascertain the existence of products meeting the identified need.
- Identify the unique selling points of your proposed product based on customer requirements to ensure its viability.
- Gather user needs to inform the design process.
- Analyze the typical user interactions that would occur with the product, whether it is a new device or an existing one.
Phase 2 – Regulatory Compliance and Gathering Feedback
This pivotal stage in medical device development encompasses design refinement and regulatory adherence. The formal risk assessment procedure and the alignment of customer and regulatory prerequisites gain heightened significance during this second phase of medical device production. This jun juncture presents an opportune moment to preemptively address risks and integrate consumer needs.
Customer feedback holds immense value, underscoring the importance of actively seeking insights through surveys and interviews. Utilize these invaluable perspectives to shape your product design, complemented by comprehensive market research and competitive evaluations.
Phase 3 – Design Development and Validation
Design Development, Verification, and Validation in Medical Device Development
The third phase of medical device development focuses on design refinement, verification, and validation. Central to this stage is the meticulous control of the medical product’s design inputs and outputs. Key components of the medical device design criteria encompass product drawings, a bill of materials (BOM), specifications, work instructions, and more.
Validating and confirming your design stands as a critical element in the progression of developing a medical device product. Throughout this phase, a consistent approach to planning, design, review, and approval must be upheld. This systematic method mitigates the risks of failure and potential harm to end-users while creating a traceable record of all activities undertaken.
During this phase, it is imperative to address key questions such as:
- Formulating a manufacturing and quality plan, including considerations for outsourcing.
- Identifying the requisite test equipment.
- Defining testing methods for validation and verification.
- Determining the optimal approach to meet customer requirements.
Phase 4 – Manufacturing and Testing Phase in Medical Device Development
During the manufacturing and testing phase of medical device development, collaboration among teams of engineers, production workers, quality assurance experts, and regulatory specialists is paramount. This phase encompasses critical elements ranging from design for manufacture (DFM) to quality control.
These multidisciplinary teams collaborate to optimize the medical device design, ensuring a seamless and efficient manufacturing process. DFM aims to streamline production procedures, enhance productivity, reduce costs, and minimize the likelihood of manufacturing challenges. By integrating DFM principles throughout the design process, you can expedite the transition from prototype to mass production.
Furthermore, meticulous selection of materials with the necessary properties and compliance with regulatory requirements is crucial. Establishing a robust supply chain and identifying reliable suppliers is essential to ensure consistent material quality and availability.
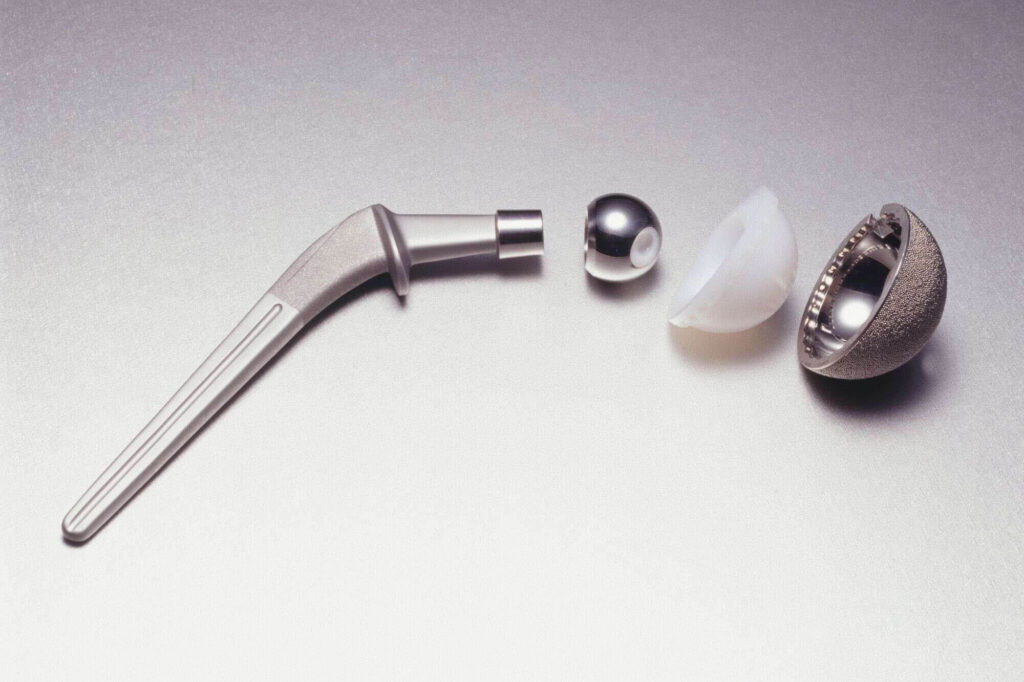
Phase 5 – Clinical Trials and Regulatory Approvals
The phase of clinical trials and regulatory approvals typically spans 1-3 years. Prior to introducing the device for commercial purchase, it is crucial to notify the medical authorities in your jurisdiction. The approval process varies based on the device’s level of risk, with Class III devices being subject to the most stringent requirements, including clinical testing before obtaining Premarket Approval (PMA).
Another key aspect of Phase 5 is the Investigational Device Exemption (IDE) process. IDE allows for the initiation of clinical trials for medical devices. Regulatory bodies may either accept the IDE, reject it, or accept it with certain restrictions. In the event of IDE rejection, studies cannot commence until the issues are rectified, and updated data is resubmitted for approval.
Phase 6 – Marketing the Product and Post-Market Phase
The final phase of medical device development encompasses the Launch and post-market phase, with the post-market phase being an ongoing process without a defined duration.
Upon receiving approval from regulatory bodies like the FDA, your products and Quality Management System (QMS) are poised for market entry. A robust production plan, validated and verified, is essential to ensure on-time delivery, adherence to budget constraints, and, most importantly, the development
Medical Device Development Timeline Based on Device Class
The development and approval of a new medical device can span several months to years, contingent upon the specific device in question. The duration of this process is intricately linked to the classification of the device.

Class I Devices
Given their low risk profile, the FDA occasionally grants approval for these devices within a week. Class I devices include items like electric toothbrushes, oxygen masks, and tongue depressors. Many Class I non-invasive devices are eligible for self-registration with the FDA.
Class II Devices
Class II devices present a moderate level of risk and encompass items such as syringes, catheters, and contact lenses. Manufacturers must conduct comparative studies with approved devices to demonstrate the safety and efficacy of their product.
Approval timelines can vary based on the specific device type submitted. For example, anesthesiology equipment typically takes an average of 245 days for approval, while toxicology devices have a shorter approval period, averaging around 163 days.
Class III Devices
Class III devices represent the most invasive and high-risk category, constituting approximately 10% of all medical devices. Examples include defibrillators, medical robots, cochlear implants, and implanted prostheses. Due to their elevated risk profile, these devices undergo rigorous testing during development to obtain clearance.
Regulatory bodies like the FDA require robust scientific evidence demonstrating the safety and effectiveness of these products. Typically, Class III device clearance takes around 243 days (or approximately eight months) from submission. It’s worth noting that recent advancements have significantly reduced this waiting period.
Advantages of Developing Medical Device Prototypes

The development of medical device prototypes is crucial and advantageous as it enables the effective production of medical products. In this context, let’s delve into the advantages of prototype development within the medical sector.
Guarantees Adherence to Regulations
Prior to advancing with further development and financial commitments, creating a prototype serves as a means to assess the project’s feasibility by collecting feedback and identifying potential investors. Certain projects may necessitate additional technical exploration to validate the feasibility of conceptual ideas before transitioning to full-scale production, particularly when designs are highly innovative.
Ensuring Regulatory Compliance
In every industry, product requirements are essential, but the medical field has particularly stringent regulations. Compliance is crucial as non-compliance can result in costly penalties or legal consequences.
The development of medical devices underscores the significance of regulatory compliance. For example, during the device development stages, prototyping can identify potential FDA violations that need to be rectified before manufacturers scale up production.
Validation and Testing for Medical Device Development
Prototyping allows for the production, construction, and testing of early-stage products, enabling efficient concept validation. While complex and unique projects may require multiple iterations of medical device prototyping to achieve significant breakthroughs, the development process helps streamline the overall processes, thereby increasing the chances of success.
Obstacles in Medical Device Development During Prototyping and Production
The development of medical devices is a complex and multifaceted process that encompasses various intricate stages, from conceptualization to market introduction. While prototyping and production are crucial phases in this process, they come with their own set of challenges.

Material Selection
Selecting the appropriate material for a medical device is typically one of the initial design stages. Medical plastic materials that are non-allergenic, durable to withstand frequent washing or exposure to chemicals, are often required for medical equipment. Additionally, the material may need to be radio frequency (RF) transparent to transmit RF signals effectively.
Financial Planning and Budget Management
Managing the delicate balance between utilizing high-quality materials and manufacturing processes while adhering to budget limitations is an ongoing challenge. Developers must efficiently optimize costs without compromising the safety and effectiveness of the medical device. As a result, the transition from the prototype stage to full-scale production necessitates meticulous financial planning to prevent budget overruns.
Iterative Design Process
The process of prototyping typically entails numerous iterations aimed at refining and enhancing the design. Managing the iterative nature of the design process can be time-intensive, impacting project schedules and financial constraints. While rapid prototyping techniques can accelerate this phase, finding the right balance between speed and precision continues to pose a challenge.
Regulatory Challenges in Medical Device Development
Having a comprehensive understanding of the regulatory requirements for medical devices is vital for all stakeholders involved in the development process. Issues related to regulations often arise during the production phase.
All parties involved must educate themselves on applicable federal laws and international standards. This level of familiarity provides valuable insights and helps them prepare for potential regulatory challenges that may arise in the future.
Guidelines and Recommendations for Medical Device Development
Developing a medical device is a meticulous process with no margin for error, given its implications for human life. If you are aiming to create a medical device that not only meets but exceeds industry standards, there are several key factors to consider.

Data Generation and Storage in Medical Device Development
If your product generates or utilizes data, it is crucial to consider how this information is stored. How is access to this data granted to individuals and other machines? Should the data be encrypted for enhanced security? Collaborating with a medical device designer can help you select the optimal and secure method for collecting, storing, and sharing any data produced by your product.
Usability Testing in Medical Device Development
Comprehensive usability testing with representative end-users is essential for identifying and resolving potential issues early in the prototype development phase. This iterative approach not only improves the overall user experience but also minimizes the risk of use-related errors.
Ensuring User Safety
Above all else, a product must prioritize safety. Neglecting safety considerations can render a product burdensome and unworthy. Unfortunately, some remarkable medical devices currently in development overlook this critical factor, resulting in potentially hazardous equipment.
Cost and Time Management in Medical Device Development
Numerous challenges can emerge during the development of medical devices, ranging from material sourcing to prototyping. It is essential to meticulously assess these challenges and establish a realistic timeline and budget. By doing so, medical device development can become more cost-effective and efficient, minimizing both expenses and time constraints.
Regulatory Compliance and Risk Management in Medical Device Development

Regulatory bodies like the FDA, EMA, and global counterparts have established a plethora of regulatory guidelines and risk management protocols to ensure safety measures are upheld throughout all stages of medical device development and production.
The International Organization for Standardization (ISO), an international non-governmental organization, sets standards to ensure the effectiveness, efficiency, and quality of products, services, and systems.
For instance, ISO 13485 outlines quality management system (QMS) standards applicable to medical device manufacturers and innovators. This standard offers guidance to ensure the highest levels of product safety and quality.
In terms of risk management, manufacturers utilize methodologies such as HAZOP and FMEA. HAZOP involves a systematic examination of current operations or processes to identify potential issues or hazards related to medical device design.
On the other hand, FMEA is a structured approach to scrutinizing a device’s components, identifying potential failure points, and assessing the associated risks.
Medical Device Prototyping and Production Services by AS Prototypes
AS Prototypes, equipped with ISO 9001 certification and cutting-edge precision machining technology, specializes in prototyping and manufacturing medical devices for your product development needs. From meticulous medical material selection to tailored machining solutions, we guarantee that each product meets efficiency and affordability standards. Our comprehensive prototyping and production services, coupled with personalized engineering assistance, establish AS Prototypes as your reliable partner in medical device manufacturing. Reach out to us today to request a quote and kickstart your project!
FAQs
What is medical device R&D?
Medical Device R&D involves various critical aspects such as competency monitoring and training, standard management, and document control. Additionally, verification and validation, risk management, and usability are pivotal components in the realm of medical device R&D.
Is FDA Approval Important for Medical Devices?
Indeed, medical devices frequently require approval from regulatory bodies such as the FDA. Prior to being marketed and sold, the FDA oversees medical devices to ensure their effectiveness and safety are up to standard.